Table of Contents
Tamil Nadu Kallakurichi Hooch Tragedy
The Kallakurichi hooch tragedy was a recent incident in Tamil Nadu, India, where people died from consuming adulterated alcohol. People consumed adulterated alcohol, also known as “hooch,” which contained harmful substances. Police are investigating the source of the liquor and have made arrests. The Tamil Nadu government is cracking down on the sale of illicit liquor.
- At least 58 people died after consuming illicit liquor.
- Over 150 people were hospitalized after falling ill.
| Past Incidence Across India |
|
Alcohol in Liquor
- Liquor is differentiated by its alcohol content, with beer having about 5%, wine around 12%, and distilled spirits approximately 40% alcohol by volume.
- The primary alcohol in these beverages is ethanol, a psychoactive drug that lowers neurotransmission levels in the body, causing intoxication.
- Ethanol (C2H5OH) consists of a carbon atom bonded to three hydrogen atoms and another carbon atom, which is bonded to two hydrogen atoms and a hydroxyl group (OH–).
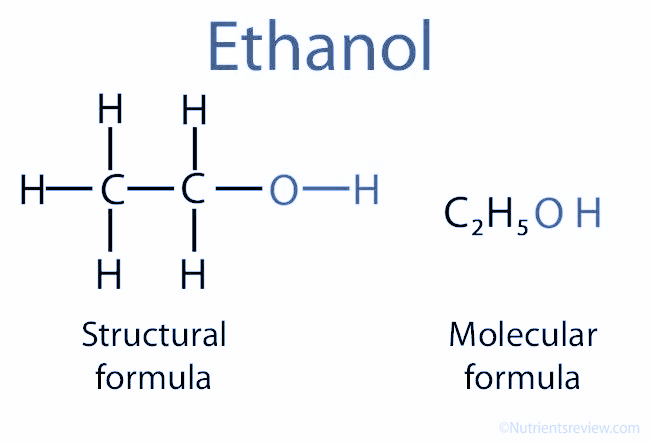
- Ethanol is metabolised in the liver and stomach by alcohol dehydrogenase (ADH) enzymes into acetaldehyde, which is then converted to acetate by aldehyde dehydrogenase (ALDH) enzyme
- The adverse effects of alcohol consumption, from hangovers to cancer, are due to acetaldehyde.
- Despite its widespread recreational use, the World Health Organization (WHO) states that no level of ethanol consumption is safe for health.
- Long-term use can lead to dependence, increase the risk of cancers and heart disease, and potentially result in death.
Spurious Liquor
- Spurious liquor contains methanol in addition to ethanol.
- Spurious liquor often involves homemade liquor with added methanol to enhance intoxicating effects and increase volume.
- The Food Safety and Standards (Alcoholic Beverages) Regulations 2018 stipulate maximum permissible methanol levels in different liquors, ranging from “absent” in coconut feni to 300 grams per 100 litres in pot-distilled spirits.
Methanol
- Methanol (CH3OH) consists of one carbon atom bonded with three hydrogen atoms and one hydroxyl group.
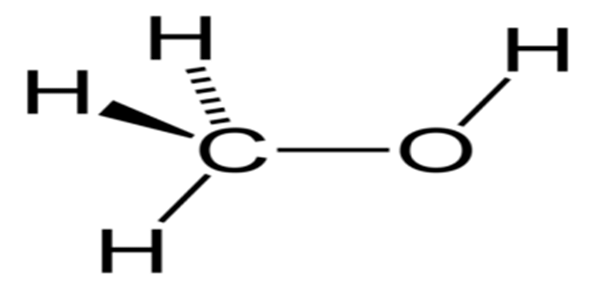
- It is included in Schedule I of the Manufacture, Storage and Import of Hazardous Chemical Rules, 1989, and its quality is governed by Indian Standard IS 517.
- Methanol is primarily produced by combining carbon monoxide and hydrogen with copper and zinc oxide catalysts at high pressure and temperature.
- Historically, methanol was produced by heating wood.
- Methanol has various industrial applications, including as a precursor to acetic acid, formaldehyde, and aromatic hydrocarbons, as well as a solvent and antifreeze.
- In Tamil Nadu, methanol manufacture, trade, storage, and sale require licences under the 1959 Rules.
How Spurious Liquor Kills?
- Methanol is deadly when ingested, with more than 1 ml per kilogram of body weight being potentially fatal.
- In the body, ADH enzymes metabolise methanol to formaldehyde, which ALDH enzymes then convert to formic acid.
- The accumulation of formic acid causes metabolic acidosis, leading to acidaemia (blood pH below 7.35), disrupting oxygen use in cells and causing the build-up of lactic acid.
- Methanol poisoning can cause cerebral edema, haemorrhage, and death.
- Also, formic acid interferes with the functioning of the optic nerve and can result in long-term visual impairment or blindness.
Treatment of Methanol Poisoning
- Methanol is absorbed via the gastrointestinal tract, with blood methanol levels peaking within 90 minutes.
- Two immediate treatments for methanol poisoning are administering pharmaceutical-grade ethanol, which competes with methanol for ADH enzymes, and fomepizole, which slows ADH enzyme action. Both treatments have limitations: fomepizole is expensive, and ethanol requires expert supervision.
- Dialysis may be used to remove methanol and formic acid salts from the blood, and folinic acid may be administered to break down formic acid.
- Both fomepizole and folinic acid are on the WHO’s list of essential medicines.
- Formic acid accumulation becomes dangerous 18-24 hours after ingestion, affecting the optic nerve, kidneys, heart, and brain.
- Ophthalmic effects are observed in 50% of methanol consumers within 24 hours.
- Co-consumption of ethanol with methanol may delay evident damage, further complicating treatment and increasing mortality.


 Utkal Divas 2025: Odisha Foundation Day ...
Utkal Divas 2025: Odisha Foundation Day ...
 List of Military Exercises of India 2024...
List of Military Exercises of India 2024...
 GPS Spoofing and Its Impact in India: A ...
GPS Spoofing and Its Impact in India: A ...





















