Table of Contents
Ozone Layer Depletion
Ozone depletion is the steady lowering of the Earth’s ozone layer in the high atmosphere as a result of human activity and the discharge of chemicals including gaseous chlorine or bromine. The thinning is mainly noticeable over Antarctica and in the polar-regions. Because it causes more ultraviolet (UV) radiation to reach the Earth’s surface, which increases the risk of skin cancer, eye cataracts, genetic damage, and immune system deterioration, ozone depletion is a significant environmental issue.
- The ozone layer in the upper atmosphere becomes thinner.
- Ozone molecules react with chlorine and bromine atoms, breaking them down.
- One chlorine molecule can destroy many ozone molecules, and they aren’t replaced quickly enough.
- Certain chemicals release these atoms when exposed to strong ultraviolet radiation.
- These are called ozone-depleting substances (ODS), Examples: Chlorofluorocarbons (CFCs), carbon tetrachloride, hydrochlorofluorocarbons (HCFCs), Bromine-containing substances: Hydro bromofluorocarbons (HBFCs), methyl bromide. Chlorofluorocarbons (CFCs).
- Chlorine does not react with ozone when it connects with another molecule
What is Ozone Layer?
The ozone layer is a region in the earth’s stratosphere that contains high concentrations of ozone and protects the earth from the harmful ultraviolet radiations of the sun. The ozone layer is a part of the upper atmosphere located about 15 to 35 km (9 to 22 miles) above Earth. It contains a high concentration of ozone molecules (O3), with about 90% of the Earth’s ozone found in the stratosphere, which extends from 10–18 km (6–11 miles) to around 50 km (30 miles) above the surface. In this layer, temperatures rise with height because the ozone absorbs sunlight. The ozone layer blocks almost all harmful solar radiation, especially certain types of ultraviolet (UV) rays that can harm or kill living things.
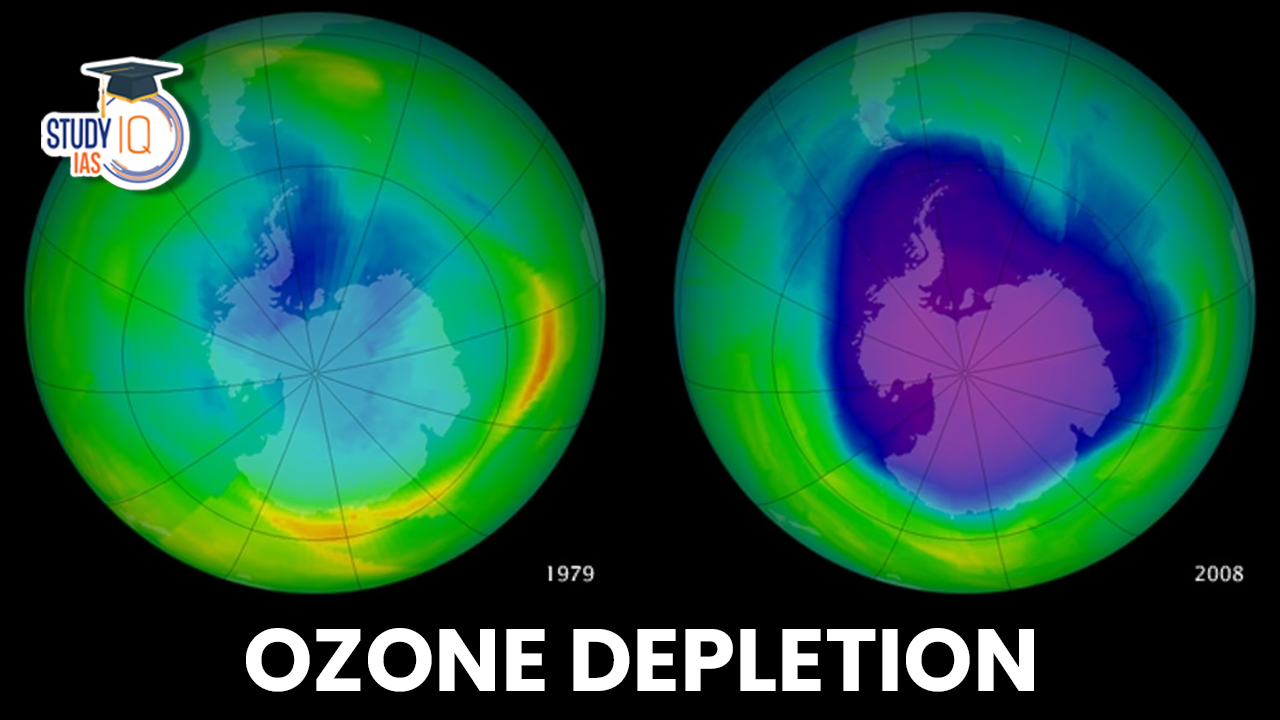
Ozone Layer Depletion status
Since 1986, there has been a big decrease in the use of ozone-depleting substances (ODS), mainly due to the 1987 Montreal Protocol by the United Nations. The biggest ozone hole ever measured was 28.4 million square kilometers in September 2000, which is nearly seven times the size of the EU. In 2023, the ozone hole is larger than it was in 2022.
Ozone Layer Depletion Causes
- Chlorofluorocarbons (CFCs): These are the main cause of ozone layer thinning. They come from solvents, spray aerosols, refrigerators, and air conditioners. UV rays break down CFCs, releasing chlorine atoms that destroy ozone.
- Unregulated Rocket Launches: Research shows that uncontrolled rocket launches can harm the ozone layer more quickly than CFCs. Without regulation, the ozone layer could lose a lot of thickness by 2050.
- Nitrogen Compounds: Substances like NO2, NO, and N2O also contribute to thinning the ozone layer.
- Natural Factors: Some natural events, like solar flares and stratospheric winds, can damage the ozone layer, but they account for only 1-2% of the loss. Volcanic eruptions can also harm the ozone layer.
Ozone Layer Depletion Effects
The loss of the ozone layer harms the environment in several ways. Here are the main effects:
Human Health: As the ozone layer gets thinner, more harmful UV radiation from the sun reaches us. This can lead to serious health issues like skin problems, cancer, sunburns, cataracts, faster aging, and weaker immune systems.
Impact on Animals: Animals exposed to UV light can develop skin and eye cancer.
Effects on Plants: Strong UV radiation can harm plants, affecting their growth, blooming, and photosynthesis. Forests also suffer from these harmful rays.
Impact on Marine Life: Plankton, which are crucial in the ocean food chain, are greatly affected by UV rays. If plankton are harmed, it disrupts the entire food chain in the water.
Ozone Layer
Many countries have programs to protect the ozone layer, but personal actions are also important. Here are some simple ways everyone can help:
Reduce Ozone-Depleting Chemicals: Use alternatives to halon fire extinguishers and avoid CFCs in refrigerators and air conditioners.
Limit Car Use: Cars produce greenhouse gases that harm the ozone layer and contribute to global warming. Try to drive less.
Choose Green Cleaning Products: Many cleaning supplies release harmful chemicals like chlorine and bromine. Use natural alternatives to help the environment.
Ban Nitrous Oxide: Governments should prohibit harmful nitrous oxide, which damages the ozone layer. People should also be educated about its negative effects and avoid products that release it.
Montreal Protocol
The Montreal Protocol, signed in 1987, was the first major international agreement aimed at stopping the production and use of chemicals that harm the ozone layer. With continued global cooperation, the ozone layer is expected to recover over time. The goal of the Montreal Protocol is to reduce harmful chemicals in the atmosphere and to stop their use, production, and import to protect the ozone layer.

 Coconut Oil Taxation in India
Coconut Oil Taxation in India
 India-made Solar Photovoltaic (PV) Cells...
India-made Solar Photovoltaic (PV) Cells...
 Tree Census: Supreme Court Ruling on Tre...
Tree Census: Supreme Court Ruling on Tre...




















