Table of Contents
Context: The pharmacy of the global South is facing a crisis of reputation.
Reasons
- Cough syrups made by pharmaceutical companies based in India, which had unacceptable amounts of diethylene glycol and/or ethylene glycol, killed 66 children in Gambia, 65 children in Uzbekistan in 2022, and 12 children in Cameroon in 2023.
- India-made eye drops contaminated by drug-resistant bacteria killed three persons and blinded eight in the U.S., again in 2023.
| Facts |
|
Why Generic Medicines Are Gaining Popularity?
- Cost-Effectiveness: Generic drugs are typically 30% to 80% less expensive than their branded counterparts, as they bypass the extensive R&D and marketing expenses associated with new drugs.
- Eg., In 2022, the U.S. healthcare system saved $408 billion through the use of generic and biosimilar medicines.
- Patent Expirations: Branded drugs are protected by patents for a limited time, after which generic manufacturers can produce equivalent versions, increasing competition and reducing prices.
- Eg., Between 2023 and 2030, patents for 169 commercialized drugs are set to expire, potentially opening the market for more generic alternatives.
- Growing Burden of Chronic Diseases: The global rise in chronic conditions such as diabetes and cardiovascular diseases necessitates affordable, long-term treatments.
- Generic medicines provide cost-effective options, ensuring that essential medications remain accessible to patients worldwide.
- Government Policies & Healthcare Reforms: Many governments promote generic drug use to reduce healthcare costs.
- Eg., the U.S. FDA’s Generic Drug Program expedites approvals, contributing to significant consumer savings annually
- Increasing Awareness & Physician Acceptance: Enhanced education and stringent regulatory standards have improved confidence in the quality and efficacy of generic drugs.
- Eg., In the U.S., 90% of prescriptions filled are for generic drugs, yet they account for only 17.5% of prescription drug spending, highlighting their cost-effectiveness.
Why India Dominates Generic Medicine Market?
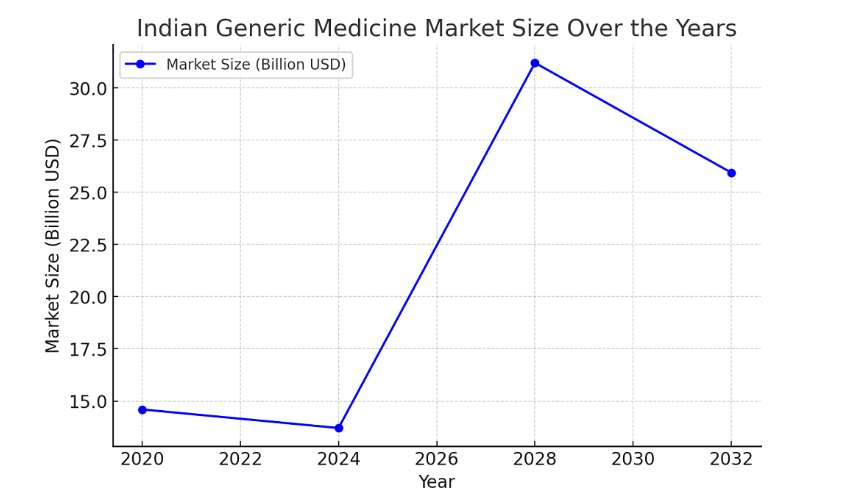
- Robust Pharmaceutical Manufacturing Infrastructure: India boasts over 670 U.S. FDA-approved manufacturing facilities, the highest number outside the United States, ensuring adherence to international quality standards.
- Cost-Effective Production: The availability of skilled labour and low production costs enable Indian manufacturers to produce high-quality generic medicines at competitive prices, making them attractive in global markets.
- Eg., Generic drugs produced in India are typically 80-90% cheaper than their branded counterparts
- Strong Export Performance: In the fiscal year 2022-2023, India’s pharmaceutical exports reached $25.3 billion, with the United States being a significant market, accounting for nearly 31% of these exports.
- Favorable Regulatory Environment: A well-established regulatory framework supports the growth of the generic drug industry, facilitating the production and export of medications that meet global standards.
- Strategic Focus on Generics: Approximately 70% of India’s pharmaceutical revenue comes from generic drugs, highlighting the industry’s strategic emphasis on this segment.
Challenges for Indian Generic Drug Manufacturers
Regulatory and Compliance Challenges
Indian manufacturers must comply with strict regulatory standards in key markets like the U.S. and EU.
- Frequent U.S. FDA inspections result in import bans or warning letters due to GMP violations, affecting exports.
- EU regulations require significant quality control and research investments to meet compliance standards.
- Adapting to evolving global regulatory frameworks increases operational costs.
Challenges in R&D and Innovation
Developing complex generics and biosimilars requires heavy investments in research and clinical trials.
- Complex drug formulations, such as biologics, demand advanced R&D capabilities and regulatory approvals.
- While Indian companies like Biocon and Dr. Reddy’s lead in biosimilars, the high cost and time-intensive nature of R&D remain a challenge.
Supply Chain and Manufacturing Complexities
Dependence on China for Active Pharmaceutical Ingredients (APIs) creates supply chain vulnerabilities.
- Disruptions, such as those seen during the COVID-19 pandemic, impact production and exports.
- Maintaining stringent quality control across manufacturing and logistics is crucial to prevent contamination and regulatory action.
Way Forward
- Strengthen Quality Control: Implement stricter quality checks, promote Good Manufacturing Practices (GMP), and enhance regulatory oversight.
- Improve Drug Distribution: Develop better supply chain infrastructure, especially in rural areas, using technology-driven tracking and inventory management.
- Promote Generic Prescriptions: Encourage doctors to prescribe generics by enforcing prescription guidelines and limiting undue pharmaceutical influence.
- Build Public Trust: Launch awareness campaigns to educate patients and professionals on the efficacy and safety of generics.
- Enhance Pharmacovigilance: Strengthen post-marketing surveillance, reporting systems, and adverse drug reaction monitoring.
- Simplify Regulatory Processes: Streamline approval mechanisms for generics to reduce delays while ensuring compliance with global standards.
- Increase Awareness Initiatives: Conduct training programs for healthcare providers and public outreach campaigns to address misconceptions.
- Tackle Patent Challenge: Strengthen legal mechanisms to prevent unfair patent extensions and support faster entry of generics.
- Ensure Sustainable Pricing: Implement fair pricing policies, encourage government procurement of generics, and promote competition while maintaining profitability.


 AI and its Regulation in India, Limitati...
AI and its Regulation in India, Limitati...
 Tuberculosis (TB), Symptoms, Causes and ...
Tuberculosis (TB), Symptoms, Causes and ...
 Silicon Photonics Enables Low-power AI A...
Silicon Photonics Enables Low-power AI A...