Table of Contents
Context
- Singapore, Hong Kong, the United States, Maldives, New Zealand, Bangladesh, and Australia are investigating contamination in spice mixes from Indian brands MDH and Everest.
- The investigations focus on the presence of ethylene oxide (EtO), a toxic chemical, in the spice mixes beyond permissible limits.
Indian Spices and Issue of Food Safety Background
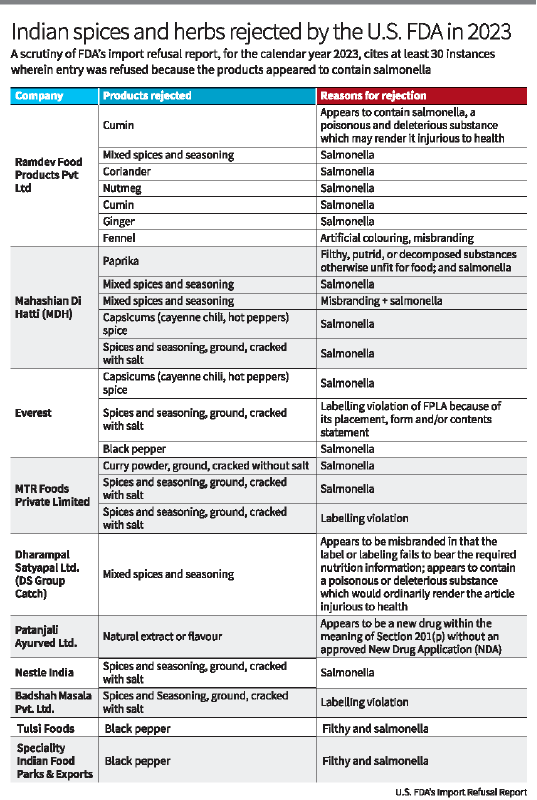
- The Centre for Food Safety in Hong Kong suspended sales of three MDH spice blends (Madras curry powder, Sambhar masala, Curry powder masala) and Everest Fish curry masala due to high levels of EtO.
- Shortly after Hong Kong, Singapore recalled an Everest spice mix, declaring it unfit for human consumption.
- MDH has denied the allegations, claiming they neither use nor have received any communication about EtO contamination from Indian authorities.
| History of U.S. Rejections of Indian Food Products |
|
Health Risks of Ethylene Oxide
- EtO is a colourless, flammable gas used for sterilisation in medical settings and industries.
- Improper use in food can leave residues, potentially forming toxic and carcinogenic compounds.
- Long-term exposure to EtO has been linked to cancers like lymphoma and leukaemia.
Food Safety Concerns in India
- Stringent Laws, Weak Implementation: Despite strong food safety regulations, consistent challenges plague the industry.
- Traceability Issues: India’s diverse food system, poor record-keeping, and intentional fraud make it difficult to track ingredients and identify potential risks throughout the supply chain. This is especially true for smaller businesses with limited resources.
- Lack of Testing Labs: At least 10 states/territories lack government-approved food testing labs, as mandated under the Food Safety and Standards Act 2006.
- Uneven Distribution and Resource Constraints: As per the FSSAI Annual Report of 2021-22 existing labs are unevenly distributed across regions and often have insufficient number of food safety officers and were found to operate ineffectively due to resource constraints.
- Transparency Concerns: A lack of transparency in FSSAI’s operations hinders efforts to meet safety standards and build trust with consumers.
Potential Impact of Spice Contamination Allegations on India
- Economic Risk to Spice Exports: The Global Trade Research Initiative (GTRI) highlighted that nearly $700 million in spice exports to key markets are at risk, potentially affecting over half of India’s total spice exports due to regulatory actions by various countries, posing a significant threat to the integrity and future of India’s spice trade.
- Potential Consequences of Regulatory Actions: GTRI noted that if Chinese regulators, along with ASEAN members influenced by Singapore, tighten their standards similar to Hong Kong, it could lead to a severe decline in Indian spice exports, estimated at $2.17 billion, or about 51.1% of India’s global spice exports.
- Further Risks from European Union: GTRI also warned that if the European Union, known for frequently rejecting Indian spice consignments due to quality issues, intensifies its regulatory measures, the losses could escalate by an additional $2.5 billion, totaling 58.8% of India’s global spice exports.
- Impact on Small Businesses and Farmers: General Secretary of All-India Kisan Sabha expressed concerns that the scrutiny on spice exports could cast doubts on other small companies and cooperatives.
- He warned that farmers could suffer financially if these events lead to reduced payments for their crops, especially since there are precedents of companies paying less even during profitable times.
India’s Response
- Mandatory Testing: The Spices Board of India announced mandatory testing for all spice consignments destined for the countries that raised concerns, specifically Singapore and Hong Kong.
- Technical Collaboration: Efforts were made to gather technical details and analytical reports from the relevant international food and drug regulatory agencies to better understand the scope of the contamination.
- Guidelines and Circulars: The Spices Board issued guidelines to spice exporters on preventing EtO contamination.
- These guidelines covered testing norms at both raw material and final product stages, proper storage of products treated with EtO, and the use of alternative methods to reduce reliance on chemical compounds.
- Inspections and Corrective Measures: The Board proposed corrective measures and initiated inspections to ensure that exporters adhere to the relevant safety standards.
- FSSAI’s Involvement: The Food Safety and Standards Authority of India (FSSAI) directed state regulators to collect samples of major spice brands, including MDH and Everest, to test for the presence of EtO.
Way Ahead
- Implement stricter safety checks for spices to detect and control EtO use.
- Ensure proper enforcement of existing food safety regulations.
- Update food safety standards to align with global practices.
- Improve communication and information flow within the food industry to enhance compliance with regulations.
| Related Information |
| Ethylene glycol, an ingredient which was found in Indian-made cough syrups that were linked to the deaths of more than 300 children in Cameroon, Gambia, Indonesia and Uzbekistan. |


 Pariksha pe Charcha 2025, Overview, Even...
Pariksha pe Charcha 2025, Overview, Even...
 National Policy on Framework on Agricult...
National Policy on Framework on Agricult...
 How Scientists used Scotch tape to Creat...
How Scientists used Scotch tape to Creat...




















