Table of Contents
The Haber-Bosch Process
- The Haber-Bosch process was developed by chemist Fritz Haber and engineers Robert Le Rossignol and Friedrich Kirchenbauer.
- It is a method used to make ammonia (NH₃), which is the main ingredient in many fertilisers.
- The process is essential because plants need nitrogen to grow, but they can’t use the nitrogen in the air directly. The Haber-Bosch process converts that nitrogen into a form plants can use.
- How it works:
- Nitrogen (N₂) from the air is combined with hydrogen (H₂) under very high pressure and moderate heat.
- A special substance called a catalyst (usually iron) helps speed up the reaction.
- This creates ammonia, which can be used to fertilize crops and boost food production.
Nitrogen Molecule
- Nitrogen (N₂) is abundant in the Earth’s atmosphere, with eight metric tonnes of nitrogen per square meter on Earth’s surface.
- In its molecular form, nitrogen atoms are bonded by a triple bond (N≡N), which makes nitrogen inert and almost unusable directly by living organisms.
- The triple bond requires 946 kJ/mol of energy to break, making it extremely difficult to convert nitrogen into a reactive form.
Nitrogen’s Role in Biology
- When the nitrogen bond is broken, it can form compounds like ammonia (NH₃), ammonium (NH₄⁺), or nitrates (NO₃⁻), which are collectively known as reactive nitrogen.
- Plants require these forms of nitrogen for synthesising essential biomolecules such as enzymes, proteins, and amino acids. Healthy plants have around 3-4% nitrogen in their tissues.
Availability of Nitrogen in Nature
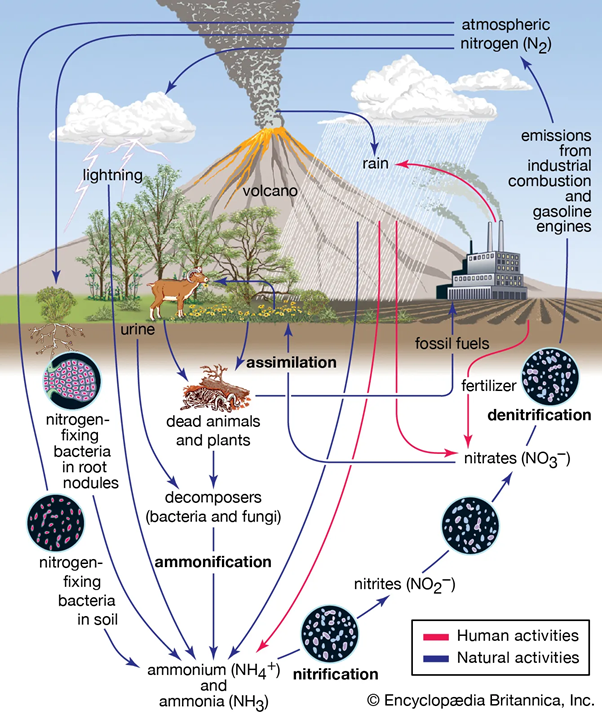
- Lightning can generate enough energy to break nitrogen bonds, creating nitrogen oxides (NO, NO₂), which react with water to form nitric and nitrous acids that fertilise soil during rainfall.
- Certain bacteria like Azotobacter and symbiotic microorganisms like Rhizobia in legumes naturally convert atmospheric nitrogen into reactive nitrogen.
- Azolla, an aquatic fern with Anabaena azollae bacteria, is another natural nitrogen converter.
The Nitrogen Cycle
| Environmental Concerns with Nitrogen Fertilisers |
|


 GPS Spoofing and Its Impact in India: A ...
GPS Spoofing and Its Impact in India: A ...
 Amrit Gyaan Kosh Portal: A Comprehensive...
Amrit Gyaan Kosh Portal: A Comprehensive...
 UpLink Initiative: Launched by World Eco...
UpLink Initiative: Launched by World Eco...





















