Table of Contents
Data Exclusivity – FTA
Context: India has rejected the demand of the four European nations EFTA bloc for inclusion of a ‘data exclusivity’ provision in proposed free trade agreements.
Background
- EU and EFTA Demands: Since 2008, the European Union and the European Free Trade Association (EFTA) countries (Switzerland, Norway, Iceland, Lichtenstein) have been pushing for data exclusivity clauses in trade negotiations with India.
- Swiss Pharmaceutical Companies: Switzerland, known for its major pharmaceutical firms, has seen some of these companies engage in legal battles in India over generic drugs.
- Leaked Draft Concerns: A leaked draft of the Trade and Economic Partnership Agreement (TEPA) suggested the presence of a data exclusivity clause, raising concerns despite advanced stage negotiations between India and EFTA.
India’s Stance
- India has consistently rejected these demands to protect its generic drug industry.
- Indian officials have affirmed their support for the domestic generic drug industry, emphasising its importance for the country’s health sector and export economy.
- India vows to safeguard the interests of its generic drug industry in all free trade agreements, ensuring no compromise on this front.
Data Exclusivity: An Overview
- Definition: Data exclusivity protects the research data generated by innovator companies, demonstrating the effectiveness of their products, from being used by competitors.
- Importance in Pharmaceuticals: In the pharmaceutical industry, companies conduct costly international clinical trials to establish the safety and efficacy of new medications.
- Impact on Market Competition: Exclusive rights to this data enable original drug manufacturers to block competitors from obtaining marketing approval for generic versions of their drugs during the exclusivity period.
Concerns Regarding Data Exclusivity
- Advocacy by Developed Countries: Developed nations push for data exclusivity in developing countries to compensate for drug development investments and R&D costs, similar to patent protections.
- Impact on Generics: Data exclusivity prevents generic drug market entry, affecting public health by limiting access to affordable medicines.
- Pharmaceutical Strategy: Drug companies favour data exclusivity to extend their market monopolies beyond the standard 20-year patent period or to obtain exclusivity for drugs without patents, thereby stalling competition from generics.
- Requirement for Generic Manufacturers: Generic manufacturers are compelled to conduct original clinical trials anew during the data exclusivity period instead of simply demonstrating bioequivalence, posing a considerable obstacle.
- Consequences of Data Exclusivity: The introduction of generics is delayed in jurisdictions with data exclusivity due to the prohibitive costs and logistical hurdles of repeating clinical trials.
- Price and Access Implications: Data exclusivity contributes to maintaining high drug prices by curtailing generic competition, rendering medications unaffordable for many.
- Ethical Concerns: The requirement for generics to undergo fresh clinical trials raises ethical questions due to the redundancy of human testing.
India’s Positions
- Potential Effect in India: Data exclusivity in India could challenge Section 3(d) of the Patents Act, which bars patent ever-greening, by allowing exclusive rights for non-patented drugs.
- India’s Position: Contrary to Western practices, India does not enforce data exclusivity, permitting the approval of bioequivalent generics utilising trial data that is already available to the public.
We’re now on WhatsApp. Click to Join
Global Pulses Conference
Context: The Global Pulses Conference, an annual meeting of pulse producers, processors and traders, has suggested India to augment production of pulses to meet the nutritional requirements.
About Global Pulses Conference
- Organised By: National Agricultural Cooperative Marketing Federation of India Ltd. (NAFED) and the Global Pulse Confederation (GPC).
- Aim: To facilitate knowledge sharing among experts, stakeholders, and policymakers.
- Previous Editions:
- 2023: Sydney, Australia
- 2022: Dubai
About Global Pulse Confederation (GPC)
- Previous Name: Initially known as CICILS IPTIC.
- Representation: The Global Pulse Confederation (GPC) represents various sectors in the pulse industry including growers, researchers, logistics, traders, exporters, importers, governmental entities, multilateral organisations, processors, canners, and consumers.
- Membership Composition: Comprises 24 national associations and over 600 members from the private sector.
- Headquarters: Dubai.
Pulses Production In India
- Global Standing in Pulses: India is the world’s largest producer (25%), consumer (27%), and importer (14%) of pulses.
- Contribution to Agriculture:
- Pulses occupy about 20% of the total area under food grain cultivation.
- They contribute approximately 7-10% to India’s total foodgrain production.
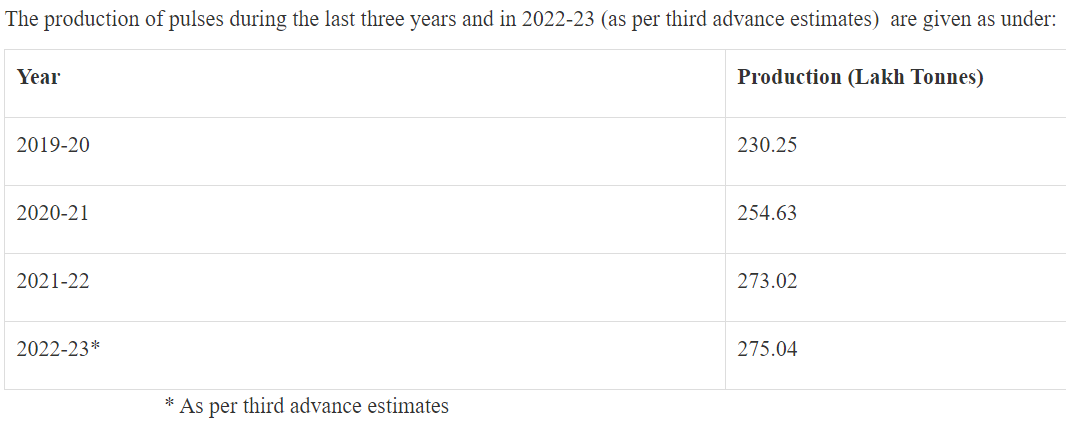
- Seasonal Production:
- Pulses are cultivated during both Kharif and Rabi seasons.
- Over 60% of India’s pulse production comes from the Rabi season.
- Leading Producing States: The top states for pulse production are Madhya Pradesh, Maharashtra, Rajasthan, Uttar Pradesh, and Karnataka.


 Utkal Divas 2025: Odisha Foundation Day ...
Utkal Divas 2025: Odisha Foundation Day ...
 List of Military Exercises of India 2024...
List of Military Exercises of India 2024...
 GPS Spoofing and Its Impact in India: A ...
GPS Spoofing and Its Impact in India: A ...





















